What is BioMedical Waste Authorisation?
Bio-medical Waste Authorisation is an authorisation issued under the provisions of Biomedical Wastes Management Rules, 2016 for the generation, collection, reception, storage, transportation, treatment, disposal, or any other manner of handling of biomedical wastes listed in Schedule 1 of the Bio-Medical Wastes Management Rules, 2016.
Medical wastes, which are generated during the diagnosis, treatment, or immunization of human beings or animals or in research activities pertaining thereto or in the production or testing of biological, including categories mentioned in Schedule I, of the BMW rules, 2016, are called Bio-Medical Waste. Clinics, hospitals, labs, and other medical establishments that generate, collect, store, transport, and dispose of biomedical waste need to set up the requisite mechanism for the efficient disposal of generated waste either directly or through a common biomedical waste treatment and disposal facility (ies).
The Bio-Medical Waste Management Rules, 2016, have hence been notified to efficiently manage the generated bio-waste in the country. Every occupier or operating handling bio-medical waste, irrespective of quantity, is required to obtain authorization under section 10 of the Bio Medical Waste Management Rule 2016.
Untreated disposal of medical waste will cause environmental pollution, unpleasant smell, and growth and leading to the development of insects, rodents, and worms, and may lead to the transmission of diseases like typhoid, cholera, hepatitis, and AIDS through contaminated syringes and needles with an infected human.
Diseases, which spread through water, sweat, blood, body fluids, and contaminated organs, are communicable in nature and are important to prevent. Diseases like plague and rabies also spread through this medical waste, which is around the hospitals, inviting flies, insects, rodents, cats, and. Rag pickers in the hospital, sorting out the garbage, are at risk of getting tetanus and HIV infections. One of the primary responsibilities of Health administrators is to manage hospital waste in the safest and eco-friendly manner.
What are the Categories of Disposal of Biomedical Waste Authorisation?
Chemical treatment with a solution of at least 1% hypochlorite or an equivalent chemical reagent. Chemical treatment must be ensured to be free from disinfection.
* Mutilation/shredding must be done in such a way that unauthorised reuse is prevented..
** Before incineration, there will be no chemical pre-treatment. Incineration of chlorinated plastics is prohibited.
*** Only towns with populations under five lakhs and rural areas will have the option of a deep burial.
|
Option
|
Treatment & Disposal
|
Waste Category
|
|
Cat. No. 1
|
Incineration ** /deep burial ***
|
Anatomical Waste from Humans (human tissues, organs, body parts)
|
|
Cat. No. 2
|
Incineration ** /deep burial
|
Animal waste includes animal tissues, organs, carcasses, bleeding parts, fluid, blood, and experimental animals used in research, as well as garbage generated by veterinary hospitals and colleges, discharge from hospitals, and animal houses.
|
|
Cat. No. 3
|
Local autoclaving/ micro waving/ incineration **
|
Waste from microbiology and biotechnology (wastes from laboratory cultures, stocks or specimens of micro-organisms live or attenuated vaccines, human and animal cell culture used in research and infectious agents from research and industrial laboratories, wastes from production of biological, toxins, dishes and devices used for transfer of cultures)
|
|
Cat. No. 4
|
Disinfections (chemical treatment /autoclaving/micro waving and mutilation shredding *
|
Sharps (needles, syringes, scalpels, blades, glass, etc.) that could cause puncture and cuts should be thrown away. This includes sharps that have been used as well as those that have not been used.)
|
|
Cat. No. 5
|
Incineration ** / destruction & drugs disposal in secured landfills
|
Medicines that have been thrown away and cytotoxic drugs (wastes comprising of outdated, contaminated and discarded medicines)
|
|
Cat. No. 6
|
Incineration **, autoclaving/micro waving
|
Disposal of Solid Waste (Items contaminated with blood and body fluids including cotton, dressings, soiled plaster casts, line beddings, other material contaminated with blood)
|
|
Cat. No. 7
|
Disinfections by chemical treatment autoclaving/micro waving & mutilation shredding. *
|
Disposal of Solid Waste (waste generated from disposable items other than the waste sharps such as tubing, catheters, intravenous sets etc.)
|
|
Cat. No. 8
|
Disinfections by chemical treatment and discharge into drain
|
Waste in Liquid Form (waste generated from laboratory & washing, cleaning , house-keeping and disinfecting activities)
|
|
Cat. No. 9
|
Disposal in municipal landfill
|
Ash from incinerators (ash from incineration of any bio-medical waste)
|
|
Cat. No. 10
|
Chemical treatment & discharge into drain for liquid & secured landfill for solids
|
Waste Chemicals (chemicals used in production of biological, chemicals, used in disinfect ion, as insecticides, etc)
|
The separation of bio-medical waste is the most important aspect of hospital waste management. Segregation of trash should take place on the premises of the hospital or nursing home. The colour coding, container type, and suggested treatment solutions for each waste category are listed below.
What are the Documents required for the Authorization?
âž² ID and Address proof of authorized person of the medical establishment.
âž² PAN card copy of the authorized person of the medical establishment.
âž² Address proof of establishment like a rent agreement, electricity bill etc
âž² MCD license
âž² Waste collection agreement
âž² Company’s PAN, MOA, COI if the establishment is in of company form.
âž² Board declaration for authorized signatory. (if the establishment is in of company form)
Who needs Biomedical Waste Authorisation?
In accordance with the provisions of the Bio-medical Waste Management Rules, 2016, every occupier/operator handling or collecting bio-medical waste is required to obtain Bio-medical Waste Authorisation, irrespective of quantity:
âž² prior to establishment,
âž² before undertaking any expansion or change in activity or infrastructure, and
âž² at the time of change in name or ownership.
Following is a list of some institutions which mandatorily need to obtain Biomedical Waste Authorisation
âž² Government hospitals/private hospitals/nursing homes/ dispensaries and Clinics.
âž² Primary health centres.
âž² Medical colleges and research centres/ paramedic services.
âž² Veterinary colleges and animal research centres.
âž² Blood banks/mortuaries/autopsy centres.
âž² Biotechnology institutions.
âž² Production units.
âž² Diagnostic Centres
What is Bio-Medical Waste?
Biomedical waste also known as hospital waste implies any waste which contains infectious or potentially infectious material(s).This kind of waste generally includes waste produced by hospitals, clinics (dental clinics, physicians clinic etc), medical research laboratories as well as pathology laboratories.
Bio-medical Waste (Management & Handling) Rules, 1998 have defined Bio-Medical as a type of bio-waste because of the possibility that it might contain hold bodily fluids like blood or other contaminants prescribed thereunder. These rules were prescribed by the Ministry of Environment & Forests (MoEF) which was formed under the Environment (Protection) Act, 1986.
Examples of Bio-Medical Waste are:
1. Discarded blood
2. Waste Sharps include potentially contaminated (used or unused) needles, scalpels, lancets and other devices capable of penetrating skin etc.
3. Unwanted microbiological cultures and stocks
4. Identifiable body parts
5. Other human or animal tissue
6. Used bandages and dressings
7. Discarded gloves
8. Other medical supplies that may have been in contact with blood and bodily fluids
9. Laboratory waste which has any of the characteristics described above.
FAQs
Q. What is biomedical waste?
A. Means any waste created during the diagnosis, treatment, or immunization of humans or animals or related research activities, or in the manufacture or testing of biological or health camps, including the categories referred to in Schedule I annexed to those laws.
Q. To whom is this authorization applicable?
A. These rules shall apply to all persons who, including hospitals, nursing homes, clinics, pharmacies, veterinary institutions, animal households, pathological laboratories, blood banks, Ayush hospitals, clinical establishments, research or educational institutions, health camps, medical or surgical centers, etc. produce, process, collect, store, transport, treat, disposal or handle bio-medical waste in any type,
Q. What are the categories of Biomedical Waste?
1. YELLOW CATEGORY
a. Human Anatomical Waste:
b. Animal Anatomical Waste
c. Soiled Waste
. Expired and discarded medicines
. Chemical solid waste
. Chemical liquid waste
. Discarded linen, mattresses, bedding contaminated with blood or body fluid
h. Microbiology, Biotechnology, and other clinical laboratory waste
2. RED CATEGORY
Contaminated Waste (Recyclable)
3. WHITECATEGORY
Waste sharps, including Metals
4. BLUE CATEGORY
Glassware, Metallic Body Implants
Q. What are the documents required for filing an Online application for Bio-Medical Waste Management Authorisation?
Documents required for the Online application of Bio-Medical Waste Authorisation are as follows:
a. Company/Partnership/LLP PAN card copy
b. Address proof of the unit
C. Authorized person aadhar card and PAN card copy
. Agreement with the waste treatment facilitator
. Other additional documents, depending on the state authority
Q. Whether the Consent of Air and Water is required before applying for Bio Medical Authorization?
A. Yes, a Consent from the respective Pollution Control Board is necessary before applying for Bio-Medical Authorisation Online.
What is the color coding and container type prescribed for the disposal of bio-medical waste?
|
Colour Coding
|
Type of Containers
|
Waste Category
|
Treatment Options as per Schedule 1
|
|
Yellow
|
Plastic bag
|
1,2,3,6
|
Incineration/deep burial
|
|
Red
|
Disinfected Container/ Plastic bag
|
3,6,7
|
Autoclaving/Micro waving/ Chemical Treatment
|
|
Blue/ White translucent
|
Plastic bag/puncture proof container
|
4,7
|
Autoclaving/Micro waving/ chemical treatment and destruction/shredding
|
|
Black
|
Plastic bag
|
5,9,10 (Solid)
|
Disposal in secured landfill
|
Notes:
1. Color coding of waste categories with numerous treatment choices as established in Schedule 1 will be determined based on the treatment option selected, which must be as stipulated in Schedule 1.
2. Chlorinated plastics shall not be used in the garbage collecting bags for waste kinds that require incineration.
3. Containers/bags are not required for categories 8 and 10 (liquid).
4. If disinfected locally, Category 3 does not need to be placed in containers or bags.
What are the labeling requirements for bio-medical waste?
For identification and safe management of bio-medical waste, different labels for containers and bags will be required. These labels for biomedical waste storage and transportation are as follows:
1. LABEL FOR BIO-MEDICAL WASTE CONTAINERS/BAGS


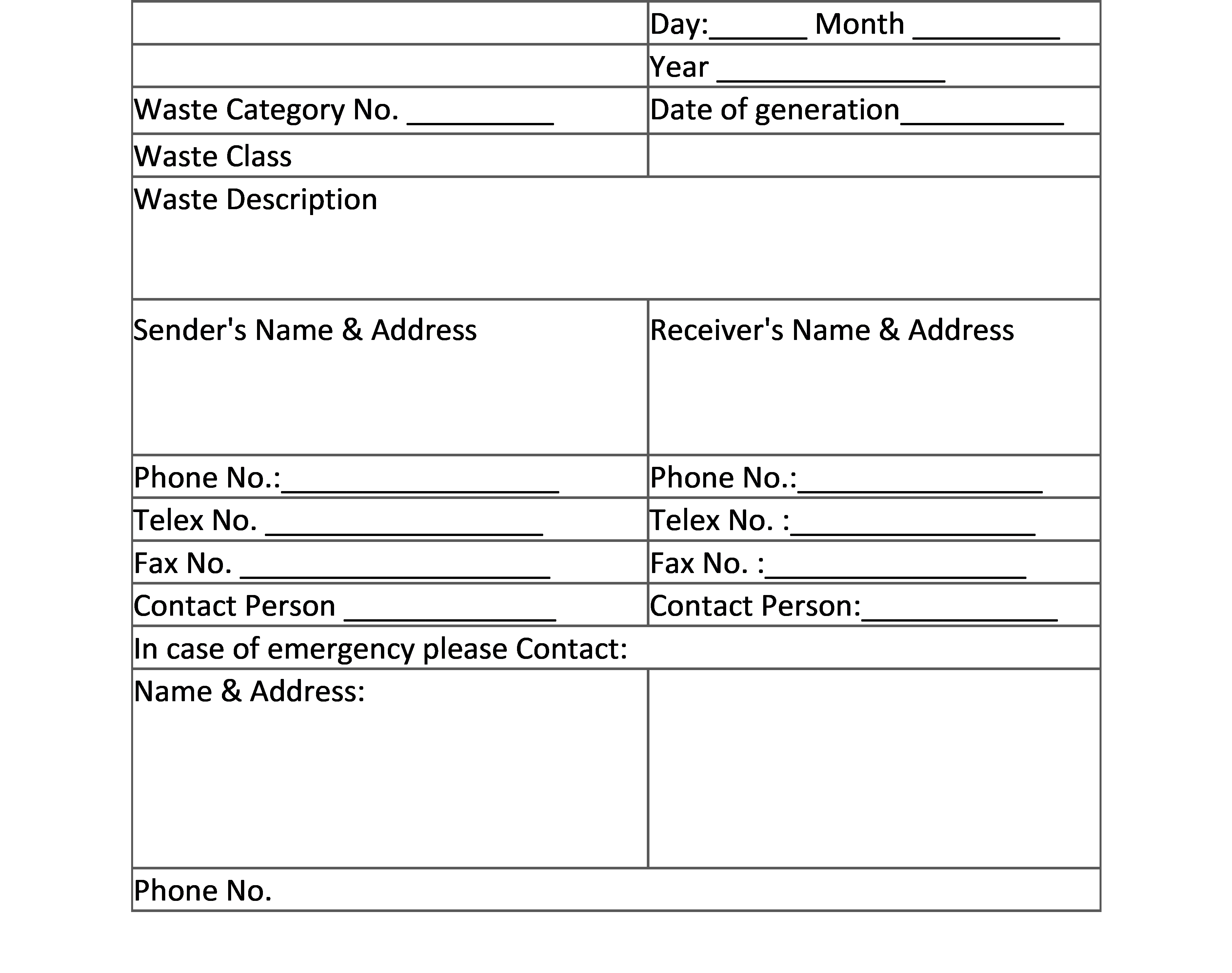
2. LABEL FOR TRANSPORT OF BIO-MEDICAL WASTE CONTAINERS/BAGS:
What is the Procedure for Bio Medical Waste Authorisation?
- Create Login on the pollution department portal
For applying applicant needs to register them self on the state pollution board’s web portal
- Apply in form II
Make an application in Form II to State Pollution Control Board, fill up all the details of the unit, all the detail of bio-medical waste generated in the unit and attach the required documents.
- Review and Payment
After filling up all the details in the form, review the form before submission and make the payment of government fees (generally the fees is of 5k but it may vary state to state)
- Application scrutinize by the department
The department will check the application and make sure that the applied application is okay with all described parameter Bio-Medical Waste (Management and Handling) Rules, 1998. In case of denial of renewal , cancellation or suspension of the authorisation by the prescribed authority the reasons shall be recorded in writing
- Issue of License
The authorisation shall be one time for non-bedded occupiers and the authorisation in such cases shall be deemed to have been granted if not objected by the prescribed authority within a period of 90 days from the date of receipt of duly completed application along with such necessary documents. In case of any change in the Bio-medical waste generation, handling ,treatment and disposal for which authorisation was earlier granted the occupier or operator shall intimate to the prescribed authority about the change or variation in the activity and shall submit a fresh application in form II for modification of the conditions of authorisation.